UChicago Medicine clinical trial leads to groundbreaking new uveal melanoma drug
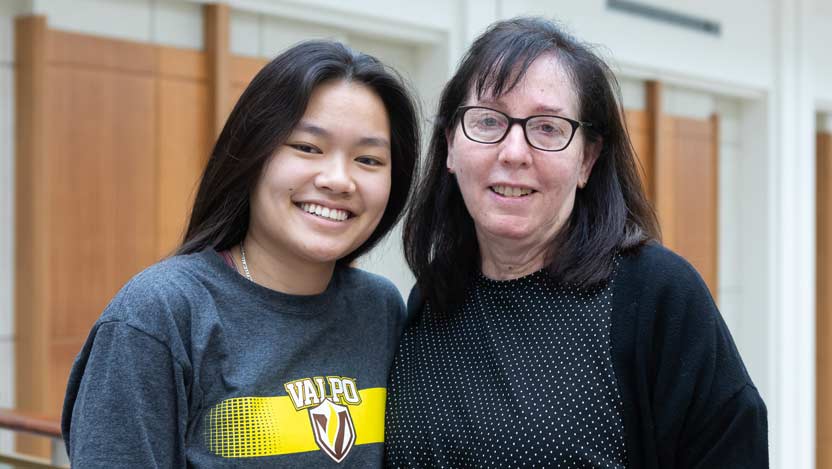
Uveal melanoma patient Kathy Huyser, right, and her daughter, Kaylee Huyser
A groundbreaking new cancer drug, tebentafusp-tebn, was melanoma, a rare type of cancer that forms in the back of the eye. It can be hard to detect, giving the cancer an opportunity to spread throughout the body.
Participants, including Huyser, received weekly intravenous (IV) infusions and blood tests, and CT scans every 12 weeks. While the drug did not help every patient in the trial, it proved to be a viable tool in fighting uveal melanoma. In Huyser’s case, it shrunk her tumors and has kept her cancer under control for four years.
Because UChicago Medicine was one of just a handful of hospitals in the United States to participate in this clinical trial, it is now one of first centers nationwide offering tebentafusp-tebn to uveal melanoma patients.
“It’s a significant advance because there were no approved drugs to treat this cancer until now,” said UChicago Medicine oncologist Thomas F. Gajewski, MD, PhD, who has dedicated his career to finding melanoma treatments and immunotherapies. “This gives us our first clue on how to treat uveal melanoma and we can keep building upon that. The hope is that we can improve the efficacy even further and expand the subset of patients who will respond well to it.”
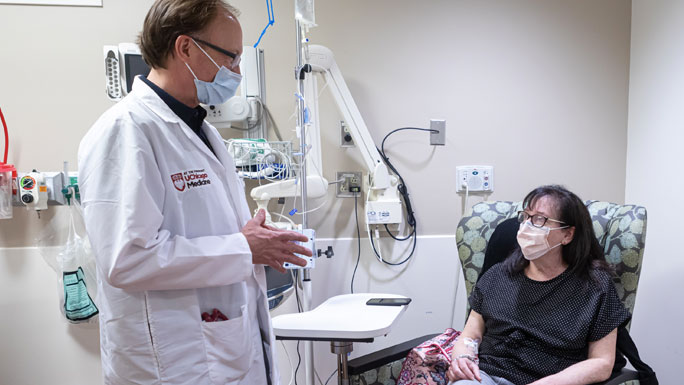
Huyser, 62, said the treatment saved her life. A single mom of two who works full-time as a neonatal intensive care unit nurse in Indiana, Huyser first noticed something was wrong 20 years ago when her vision became fuzzy and distorted. Doctors found a cancerous tumor in her right eye and it was treated with radiation. While the radiation permanently damaged the vision in her right eye, her life returned to normal for the next 15 years.
Then, five years ago, Huyser experienced sharp stomach pains. A CT scan found multiple tumors in her abdomen, liver and lungs, which biopsies confirmed to be metastatic uveal melanoma. Huyser’s doctors recommended she join the clinical trial at UChicago Medicine.
“I always felt fine. That’s the scary thing. If I hadn’t gone for a CT scan, I would have never known. It was just a fluke that I went,” she said.
Starting in December 2017, Huyser had received a weekly infusion of tebentafusp-tebn and a blood test. She hasn’t missed a single week of treatment in four years, and is usually accompanied by her dad, Hank, beloved by the UChicago Medicine staff.
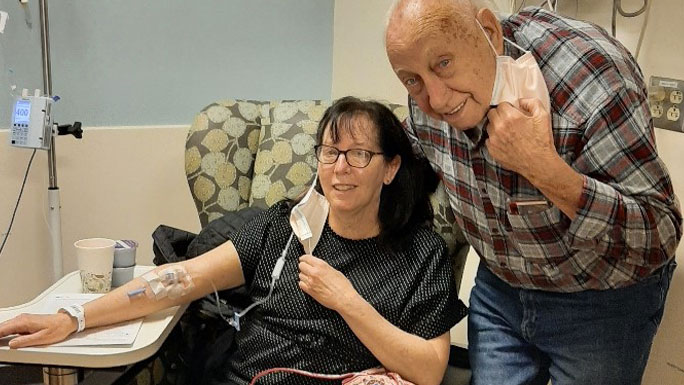
As an example of how novel this treatment is, another patient involved in the trial flew to Chicago every week from California for the tebentafusp-tebn infusion.
The only side effect Huyser experienced was that her hair and eyebrows greyed and whitened. Other than that, she’s felt completely healthy and is able to work and do all her normal activities.
“God had a plan for me to be in this trial,” Huyser said. “It’s been a miracle in my life because I can still function at 100%. It’s like this drug was just made for me. That shows how good the drug was, that people around me didn’t even know that anything was wrong.”
Huyser has grown close to the UChicago Medicine physicians and nurses who treat her each week.
“It’s like family up here,” she said. “The plan, for now, is just to keep going with the weekly treatments.”

Thomas F. Gajewski, MD, PhD
Thomas Gajewski, MD, PhD, investigates and develops new treatments for patients with melanoma. He also leads development of immune-based therapies for other cancers, using new laboratory data on how the immune system is regulated to develop novel clinical trials.
Read Dr. Gajewski's physician profile
Participate in a Cancer Clinical Trial
UChicago Medicine cancer experts are actively conducting clinical trials of new screening methods and treatments for cancer, including breakthrough approaches to harnessing the immune system to fight cancer. As a recognized leader in phase I and other early-phase clinical trials, our physician-scientists are creating new cancer treatment methods that later become the standard of care elsewhere.
Find a cancer clinical trial