Molecular imaging probes the effects of nicotine and treatments to stop smoking

Tobacco is the leading cause of preventable death in the United States. Despite its link to disease, including cancer, millions of people still use tobacco worldwide. Researchers are interested in understanding the underlying mechanisms of nicotine, the major addictive chemical found in tobacco, in order to develop more effective strategies to overcome addiction.
When nicotine enters the body, it binds to neurotransmitter receptors in the brain called nicotinic acetylcholine receptors (nAChRs) that in turn bind to the neurotransmitter acetylcholine (Ach).
Varenicline, the smoking cessation drug known as Chantix, is understood to work by activating the pleasure-producing nAChRs, making fewer available targets for nicotine to bind to, thereby curbing nicotine’s effects. Prolonged exposure to nicotine, however, causes an increased number of binding sites, a process known as “upregulation” that is important in the craving and withdrawal effects seen in nicotine addiction.
Because varenicline also has been observed to cause increased upregulation, scientists have been long puzzled about how varenicline works to reduce nicotine cravings. And because varenicline, the most effective drug of its kind, only stops smoking in about 30–40% of people who use it, more knowledge is needed to explain its relationship to nicotinic receptors.
The laboratory of William Green, PhD, professor of neurobiology, made progress on this front when they discovered that varenicline gets trapped within cells’ acidic compartments called vesicles that contain nAChRs. Chronic nicotine exposure increases the numbers of vesicles, thereby increasing varenicline trapping in neurons. Nicotine, however, does not get trapped and rapidly escapes the vesicles.
The slow release of the trapped varenicline from the vesicles reduces nAChR signaling caused by nicotine-induced upregulation.
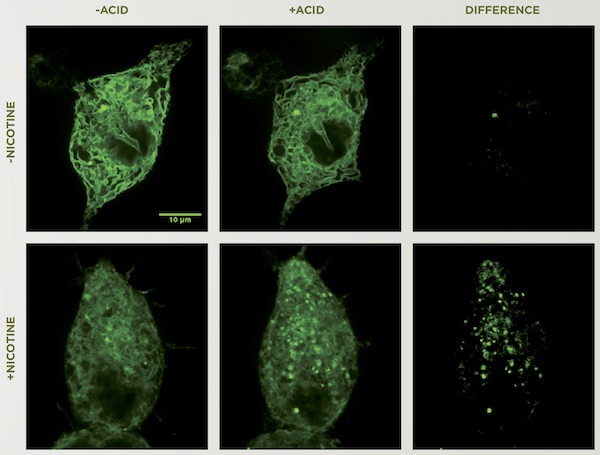
This discovery may provide a new paradigm for how varenicline works. It also leads to many other questions, such as, how can studying nAChRs’ interactions with molecules such as varenicline and nicotine be used to design more effective therapies? Novel molecular imaging approaches provide an opportunity to better understand these mechanisms at a molecular level.
Green is collaborating with Jogeshwar Mukherjee, PhD, professor of radiological sciences at the University of California–Irvine and former radiochemistry faculty member at UChicago in the 1990s, to develop positron emission tomography (PET) probes to image proteins in the brain that respond to nicotine. PET involves radioactive tracers that are injected into the body, allowing radiologists to visualize what is happening as the “glowing” molecules are processed.
Building on their hypothesis that compounds with fast kinetics are trapped less in acidic vesicles and those with slow kinetics are trapped more, the researchers will be testing different PET probes in cells and brains in animals to find the optimal probe for studying nicotine receptors. Just like nicotine and varenicline, PET probes have different properties that affect how they bind to receptors.
Green and Chen anticipate that findings from their work may be applied to the work of Andrea King, PhD, and At UChicago Medicine, we offer a wide range of lung cancer care options, including minimally invasive surgery and innovative targeted therapies, as well as clinical trials of promising treatments not widely available.
Lung Cancer Care
