Researchers find new mechanism to turn on cancer-killing T cells
Over the past decade, researchers have made great strides in the development and administration of cancer immunotherapies, which use the body’s own immune system to treat disease. However, the therapies don’t work for every person or with every type of cancer, and gaps in our understanding of exactly how the body mounts an anti-cancer immune response has slowed progress toward making them universally effective.
In a new study, researchers at the University of Chicago Medicine Comprehensive Cancer Center and the University of Amsterdam have brought insight into one crucial step in the anti-cancer immune response process: T cell priming.
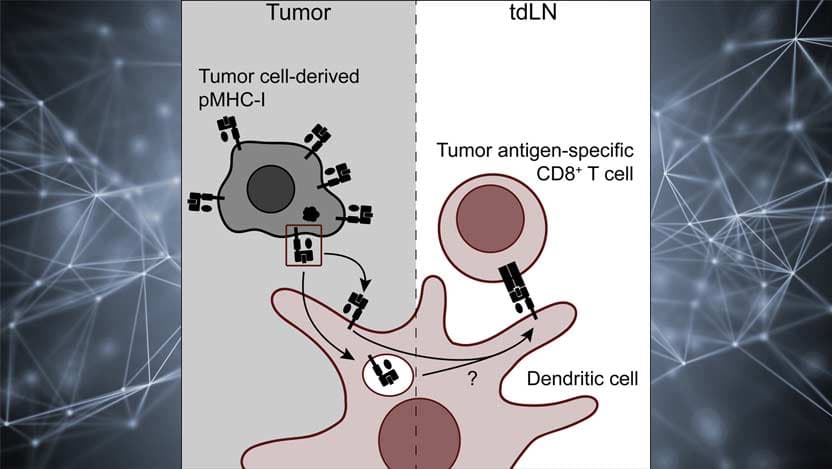
Previous studies implied that a single mechanism — antigen cross presentation — is responsible for priming T cells, the immune system’s disease fighters, to identify and attack cancer cells. The new study found that a second mechanism, known as MHC-I cross-dressing, is also effective in prompting a T cell response.
“What’s significant is that we identified a totally unique pathway whereby tumors and the immune system talk to each other,” said Justin Kline, MD, an associate professor of medicine at UChicago Medicine and an author of the study. “Knowing that this pathway exists might have implications for how we think about vaccine design in the future or how we predict which tumor antigens might be best to target.”
The study, “Dendritic cells can prime anti-tumor CD8+ T cell responses through major histocompatibility complex cross-dressing”, published May 25, 2022 in Immunity.
‘Dressing’ in molecules
Kline and his team study the role of dendritic cells in the cancer immune response. These cells alert the immune system to antigens – toxins and other foreign materials in the body – and spur T cells into action.
As noted earlier, researchers were under the assumption that antigen cross presentation was the exclusive mechanism used by dendritic cells to talk to T cells. Cross presentation happens when a dendritic cell “eats” a cancer cell and then displays what it has eaten so that T cells can see if any antigens are present.
The mechanism Kline and his team identified simply requires dendritic cells to “dress themselves” with a tumor cell’s molecules to alert T cells to the disease.
The researchers realized the potential existence of this second mechanism by an unexpected observation in the lab.
Knowing that this pathway exists might have implications for how we think about vaccine design in the future or how we predict which tumor antigens might be best to target.
“The initial discovery came about sort of serendipitously when we knocked out a specific MHC class 1 molecule on mouse tumor cell lines and found that the immune response against those tumors was significantly and negatively impacted despite the fact that cross presentation was fully intact,” said Kline. “It indicated to us that a second mechanism was operational, and we wanted to investigate it further.”
Identifying a new mechanism
To do this, the researchers genetically engineered dendritic cells in mouse models to suppress the expression of MHC-1 molecules, whose major function is to display tumor-associated antigens. They then injected the models with one of two types of cancer tumors. The first type, melanoma, has very low levels of MHC-1 molecules. The second type, leukemia, has very high levels.
Once injected, they used flow cytometry to measure the presence of MHC-1 molecules on the dendritic cells and found that the cells had taken on – or dressed themselves – in the molecules. Furthermore, they discovered that the dendritic cells of the models injected with leukemia showed significant amounts of the molecule, while the ones injected with melanoma had somewhat less.
“We knew that any MHC-1 molecule on a dendritic cell had to come from the tumor because there’s no other MHC-1 molecules in the system,” said Kline. “It showed definitively that cross-dressing was taking place.”
It was also significant that the amount of MHC-1 on the dendritic cells differed based on tumor type, Kline said. It indicates that this pathway may be more important in cancer tumors with high levels of class 1 molecules, and less important in those with low levels.
To realize any practical implications of this work, more research is necessary. Next, the team plans to investigate how MHC-1 cross-dressing happens – the molecular mechanisms behind it – and the extent to which cross-dressing can impact dendritic cells’ ability to spur T cells into action.

Additional authors on the paper include Brendan W. MacNabb, Xiufen Chen, Sravya Tumuluru, James Godfrey, Darshan N. Kasal, Jovian Yu, and Douglas E. Kline of the University of Chicago, and Marlieke L. M. Jongsma and Robbert M. Spaapen of the University of Amsterdam.
Support for the research was provided by the National Institutes of Health, the University of Chicago, and the American Association of Immunologists.

Justin Kline, MD
Justin Kline, MD, is a hematologist/oncologist who specializes in treating leukemia and lymphoma. He has a special interest in stem cell transplantation and is conducting research into overcoming the barriers to immune resistance mechanisms in tumors.
See Dr. Kline's profile