Studying the shape of a protein to learn how pregnancy makes peace with the immune system
Pregnancy presents a conundrum for the immune system. It's built to fight off any invaders with foreign DNA, from viruses and bacteria to, unfortunately, lifesaving transplanted organs. Since a fetus has DNA from both parents, the mother's immune system needs to have a way to remain tolerant of the father's foreign genes and give them a pass.
One way the immune system acknowledges the special circumstances of pregnancy is with proteins produced by genes in the major histocompatibility complex (MHC), which help the immune system identify foreign bodies. In most situations, MHC proteins on healthy cells present little pieces of proteins called peptides, which are like badges to show to the body's security guards, roaming immune T cells. If they see a human or "self" peptide, everything checks out; but if they see a peptide from a virus or foreign tissue, the guards sound the alarm and trigger an immune response to kill the cell.
But during pregnancy, the fetus doesn't express a lot of these classical MHC proteins that are recognized by T cells responding to specific peptides, perhaps as a way to avoid rejection. Instead, fetal and uterine cells express related, but slightly different, non-classical MHC proteins that regulate maternal NK (natural killer) cells that supervise the extensive changes that occur in the uterus during pregnancy.
Charles Dulberger is a PhD candidate in the department of Biochemistry and Molecular Biophysics at the University of Chicago (and Science Life contributor) who studies the three-dimensional structures of proteins that control immune responses. The shape and surface topology of proteins is important to how they function. They have unique notches and grooves that allow them to interact with other proteins or bind molecules with matching shapes, like puzzle pieces fitting together.
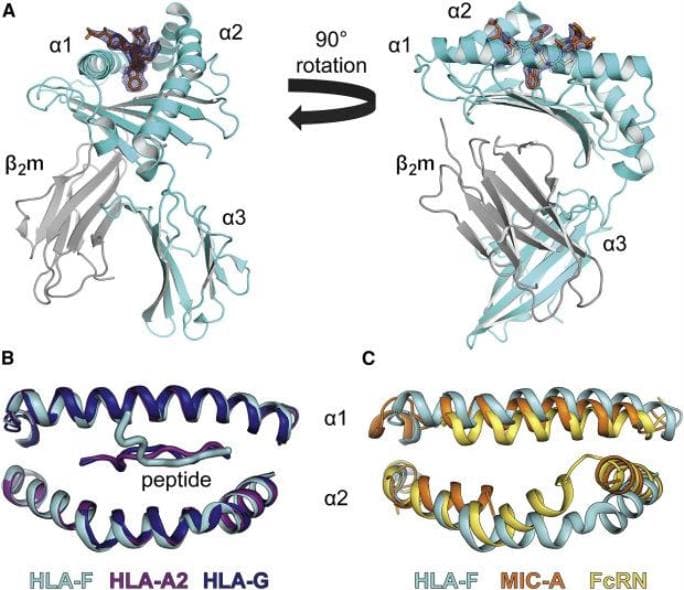
In a paper published last month in the journal Immunity, Dulberger led a team that described the structure of HLA-F, one of the specialized MHC proteins involved in pregnancy, for the first time. It hasn't been studied as thoroughly as some of its neighbors, and while it turns out that it functions in many of the same ways, it has some interesting differences that may be tied to the evolution of pregnancy in humans.
Scientists haven't been sure how HLA-F is involved in the immune system, or whether it presents peptides in the same way as other MHC proteins. Using x-ray crystallography and other biochemical methods to define its structure, Dulberger and his colleagues were able to confirm that HLA-F does bind peptides, which means it could be recognized by T cell receptors too.
"Now that we know that HLA-F can present peptides, we want to know if T cells and NK cells can use their receptors to perceive these peptides as a signal to respond to," he said. "If T cell receptors do recognize them, that opens the door for potential therapeutics that exploit or modify this interaction, because T cells are important in a lot of diseases, including many cancers."

Most MHC molecules have a well-defined, linear groove shaped to hold peptides, like a Lego piece that snaps perfectly into place. But HLA-F has a slightly different groove that is partially blocked off, so part of the peptide protrudes out of the groove. What's interesting is that these changes are only present in human and orangutan HLA-F, who notably both have nine month pregnancies-they're not there in HLA-F for chimpanzees, which have a shorter eight-month gestation.
Dulberger said that the differences may contribute to how the mother's immune system tolerates a longer pregnancy. The structure and function of HLA-F is important to understanding aspects of pregnancy, he said, but learning more about its specific shape (and of others like it) will also help us understand diseases that manipulate the immune system, like cancer.
"We can learn a lot about how cells communicate by understanding the structure of proteins and protein complexes because proteins are kind of like the eyes, ears and mouth of the cell," Dulberger said. "They sense their environment and signal to each other via protein-protein interactions."
Understanding protein structure is also really important for the development of new medicines because most drugs used in the clinic target specific proteins.
"Once you understand the molecular architecture of a protein receptor and how it binds another protein, you can design drugs that interfere with that interaction or modify it in an advantageous manner," he said. "So, you can imagine if you had a cancer that expresses HLA-F at a really high level to inhibit the immune system, you can develop an antibody or small molecule inhibitor to block that, and then the immune cells will be able to fight the cancer cells again."

