What does it mean when 2 billion people share their brain with a parasite?
Nearly one-third of the 7.25 billion people on earth, including an estimated 60 million people in the United States, are chronically infected with the protozoan Toxoplasma gondii. A working immune system can keep the parasite in check, so only a minority of those infected has significant symptoms. But when an unsuspecting woman gets infected during pregnancy and passes the parasite on to her unborn child, the consequences can be profound.
They include devastating damage to the brain, nervous system and eyes. Now there is growing evidence that some people, maybe a lot of people, have subtler symptoms triggered by the infection. Perhaps the immune response, while keeping the parasites in check, causes collateral damage elsewhere. Or maybe the parasites, which produce the neurotransmitter dopamine and interact with the cells it infects, modifies behavior of its host. We asked a leading authority on Toxoplasma gondii and toxoplasmosis, Rima McLeod, MD, professor of ophthalmology & visual science and pediatrics and medical director of the toxoplasmosis center at the University of Chicago, a big question: What do we think happens when billions of people go through life with a parasitic brain infection?For starters, what is Toxoplasma gondii?
Rima McLeod: Toxoplasma gondii is a microscopic parasite related to the parasite that causes malaria. It has several life-cycle stages.
This is an equal-opportunity parasite. It infects persons all over the world, with no respect for place, ethnicity or socioeconomic status, although host genetics do play an important role in the manifestations of infection.And what about toxoplasmosis?
Toxoplasmosis refers to the diseases caused by this parasite. I should say parasites. We are learning that genetically different parasites differ in the diseases they cause, often varying by geographic location. For example, different genetic types of parasites are present in different climate regions of the US, causing different severity of disease.
The parasite can cause disease in its acute, active form when acquired by older children and adults. Sometimes it damages the eye and occasionally heart or brain or causes enlarged lymph nodes. It is the most common infectious cause of destruction of the back of the eye. It then becomes dormant but can recrudesce in its active destructive form when the immune system is not normal, such as in persons with malignancies or AIDS or during treatment that suppresses the immune system. In that case the parasite can damage the brain or other organs.
When a woman acquires the infection for the first time while she is pregnant and transfers the parasite to her fetus, this causes a congenital parasitic infection. It can be a terrible disease when untreated or caught too late. In that setting, it can cause loss of sight, severe brain inflammation and permanent neurologic damage.
Even when there are not severe symptoms at birth, it can switch from the dormant to the active phase and cause new damage to the eye and brain. That's why screening for infection in pregnant women is so important.How do people contract the infection?
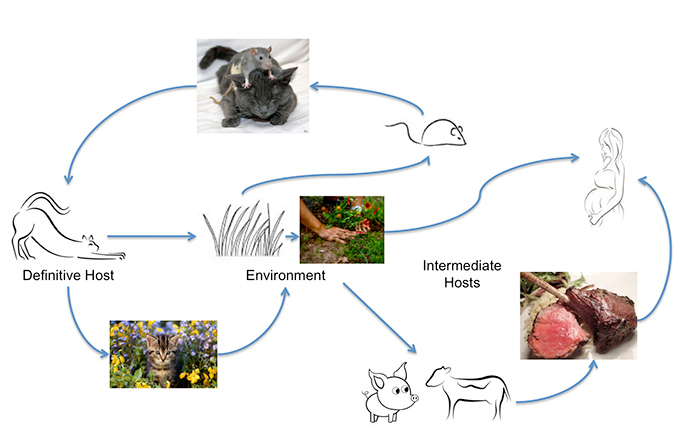
Toxoplasma gondii can infect most warm-blooded animals, including humans, but the primary host in which genetic exchange occurs is cats. Humans can acquire the infection from eating undercooked meat or by ingesting oocysts excreted by cats, on unwashed vegetables, for example, or from contaminated water. Unrecognized contact with oocysts can happen easily as an acutely infected cat can excrete up to 500 million Toxoplasma oocysts in two weeks. Even one oocyst is infectious. These survive in warm moist soil and water for a year. A study from Johns Hopkins University found that the annual oocyst burden measured in community surveys is three to 434 oocysts per square foot. Those numbers go way up, they add, "in areas where cats selectively defecate." That's why the infection is so common.
OK. That's disturbing. What happens when someone ingests a few oocysts?
T. gondii is an obligately intracellular parasite. That means that it must infect the hosts' cells to survive. The cells it prefers to live in, when in its dormant stage, are in the brain and the retina, at the back of the eye. It infects and then it hides and replicates slowly within those cells. This is a chronic, lifelong infection.
What happens to people who are immunologically normal and weren't exposed before birth who pick up parasites later on?
Many physicians have considered such latent or asymptomatic cases clinically insignificant. In my research group and laboratory, we have focused on the biology of active infections, especially in children, and how best to prevent or treat them. For more than a decade, however, there have been multiple studies of subtle, or not so subtle, changes in animal behaviors associated with latent T. gondii infection. Mice and rats with an infection, for example, lose their aversion to the smell of cats, especially to cat urine. This so-called fatal feline attraction is perilous for any rodent made fearless by infection; it makes it easier for cats to catch and eat them. But it benefits cats, which acquire an easy meal, and it helps the parasites, which gain a new reservoir. We found that chronically infected mice have additional behavioral changes, such as freezing in an open field, grooming themselves less well, loss of balance and diminished grip strength. These behaviors are typical of unhealthy aging. Brains from chronically infected mice are smaller than those from normal controls. We find parasites in cysts within neurons that had formed synapses. We also see an inflammatory process, especially within and next to the part of the brain associated with memory.
Is there a human equivalent to such behavior changes?I used to have my doubts, but I am no longer so certain that this behavioral change involves only rodents. A recent study from Stanford showed changes in immune-system molecules in blood from chronically and acutely infected pregnant women that were not present in uninfected matched controls. A group at Johns Hopkins University found subtle but specific memory deficits in young professionals who had antibody to the parasite, compared to matched controls. Several research teams have looked for effects on personality or behavior in humans. These groups have found that patients with schizophrenia or obsessive-compulsive disorder are statistically more likely to have a T. gondii infection. Infected men have slower reaction times, and more than twice as many traffic accidents. There also are associations of having antibodies to the parasite with bipolar disease, suicidal behavior, even an optimistic disposition, possibly related to the parasite's effects on dopamine. A 2012 analysis from France, where an estimated 43 percent of people carry T. gondii, concluded that men with a latent infection tend to be "more dogmatic, less confident, more jealous, less impulsive and more orderly than uninfected men. Infected women seemed warmer, more conscientious, more persistent, more insecure and more sanctimonious."
How does one measure sanctimoniousness?
I don't know what to make of that, or of the many association studies, because it is difficult to know what came first: the infection or the trait. There is one scientist who believes he is a down-to-earth first-person example. Czech researcher Jaroslav Flegr, professor at Charles University in Prague, speaks and writes about changes he noticed in his own behavior after he acquired a T. gondii infection. He has argued that latent Toxoplasma infections made men more likely to withdraw, to become hostile or antisocial, or outright 'curmudgeonly.' Infected women, on the other hand, seek solace through social bonding and nurturing. They're inclined to tend and befriend. Flegr has been known to liken them to "sex kittens." Don't ask me how that ties in with sanctimony. This type of information is in the literature but not definitively proven as a cause and effect at present.
Again, curmudgeonly behavior can be difficult to measure, yes?
Exactly, so our lab prefers to focus on understanding how T. gondii do their damage, the genes that initiate this process, as well as those that protect against Toxoplasma infection. We want to develop medicines to cure it, and to make a vaccine to prevent it. Our long-term goal has been to understand this disease in order to improve treatment and outcomes. In the process of our genetic studies, however, we have run across multiple connections between the genetic pathways that help control T. gondii infection and those implicated in neurobehavioral diseases and neurodegeneration. I increasingly suspect that this parasite-by way of the immune system's response to it and the parasites' direct interactions with host neuronal stem and differentiated cells-is influencing the same pathways as those associated with neurologic disorders, including Alzheimer's disease and possibly autism. And it points to clues about how the parasite could contribute to those diseases for some genetically susceptible people exposed to them.
How did you make the connection?
We looked closely at the genes involved in protecting a host from this parasite. A 2006 study from France identified a small region on the rat chromosome 10, now called Toxo1. This region contains about 30 genes. They found that some of those genes play a central role in preventing parasite proliferation and spread in rat cells. Curiously, the genes that protect against Toxo came from Lewis rats, a standard, docile, experimental animal bred for generations in the laboratory. The genes that fail to prevent Toxo are common in brown Norway rats, the tough guys, what many people refer to as 'sewer rats.' At that time our colleagues in France, at Universities in Grenoble and Toulouse, were having difficulty determining which genes within the region might be crucial in this. Their work in rats led us to look for similarities in the gene region in humans. This launched our studies to trace a similar set of genes, with some significant differences, to human chromosome 17. Our decades of work with patients gave us a huge head start. We follow about 250 families-each includes a mother, a father and a child infected with Toxoplasma in utero. In about 20 of those families, we have sets of twins who were exposed together. In some cases we have identical twins, both very sick, with the same manifestations of infection. In other cases, the symptoms are minimal. In cases involving non-identical twins, sometimes one of the pair is profoundly affected and the other appears normal. So we know that inheriting protective or susceptibility gene variants from mom and dad can make a difference. We are now looking closely at each of those and additional genes.
What do these genes do and what have you learned? We just published a paper on ALOX12 in the journal Infection and Immunity. We are early in the process of studying all the genes, but the results so far are fascinating. We have used a candidate gene approach. The first two genes we examined, one from either end of Toxo1 region, had variations that correlate at a high level with either susceptibility or resistance. Earlier, we had found this in the gene at the bottom of the region, known as caterpillar at the time. In this current paper, we found it in a gene at the top of the region, called ALOX12. This is a cell-death gene.
Technically, it is a lipoxygenase that adds a toxic, unstable oxygen to the 12th carbon of arachidonic acid-creating a biologically harmful molecule. Others had found evidence of ALOX12 influencing outcomes in diabetes, neurodegenerative disease and schizophrenia, but there was nothing known about its role in infectious diseases. When we tested variations of this gene from different parents and children we found that certain alleles-particular variations of four pieces of the gene-were significantly different, and that these differences were associated with susceptibility. When we reduced ALOX12, the inflammatory and cell-death activities that protect against toxoplasmosis decreased. It became likely that certain variants of this gene were more effective than others at triggering inflammation and killing infected and nearby cells and parasites. With the enzyme product of this gene there was a stronger effect in keeping the infection contained in human cells. Without the product of this gene the same cells were unable to constrain the infection. When we tested these cells with the ALOX12 gene rendered inactive, the parasites proliferated rapidly. The other gene at the other end of the region, known as caterpillar or NALP1, had a similar effect. We published this in 2011.
Is there a downside to these aggressive anti-parasite genes?
These cell-death factors found in this critical cluster of genes called Toxo1 are important in limiting this infection. But we now suspect that high activity of these genes later in life may have a harmful effect, killing not just the parasites' host cells but also innocent bystanders. This is conjecture, but it could help explain why a gene polymorphism that appears to play a role in the onset of diseases of aging, might still be preferred by evolution for its ability to prevent or control a common infection early in life, especially up through child-bearing age.
Why would these genes focus their effects on behavior or neurologic disease?
Toxoplasma infects nerve cells. Certain versions of ALOX12 and NALP1 can help an infected person mount a strong, early, protective, immune response, killing parasites, infected cells as well as nearby cells. But we also know that ALOX12 is associated with diseases related to aging and memory loss. When we utilized our findings about ALOX12 with systems-biology programs, used to map out pathways, we realized that many of these pathways were also in play in various neurodegenerative diseases, such as Alzheimer's and even schizophrenia. Some may contribute to atherosclerosis or diabetes. When this powerful immune response remains on the job it can have effects that might be harmful. More effective medicines or a vaccine could protect against the devastating diseases this parasite causes. This recent work raises the possibility that better treatments, by quickly controlling or eliminating infection, may also reduce the age-related illnesses we speculate may associate with toxoplasma for some people.
This is a big project. You seem to have a lot of partners in this effort, yes?
This has been a wonderful collaboration with colleagues in France, England and Canada and a team at the University of Kansas, along with the work of a remarkable group of young scientists in my own laboratory.

We are working in our own research programs and laboratory group and with colleagues in New Haven, the J Craig Ventner Institute, and the Institute for Systems Biology in Seattle and a number of other colleagues. We are very excited about the insights and potential to advance prevention and treatment that this work is providing.
Sign Up for Our Research & Science Newsletter
Subscribe to Science Life, our research and science e-newsletter. Each issue features articles on trending research topics and insight from UChicago Medicine scientists.
Subscribe Now
